Analytical Similarity Assessment in Biosimilar Product Development
Format:Paperback
Publisher:Taylor & Francis Ltd
Published:18th Dec '20
Currently unavailable, our supplier has not provided us a restock date
This paperback is available in another edition too:
- Hardback£175.00(9781138307339)
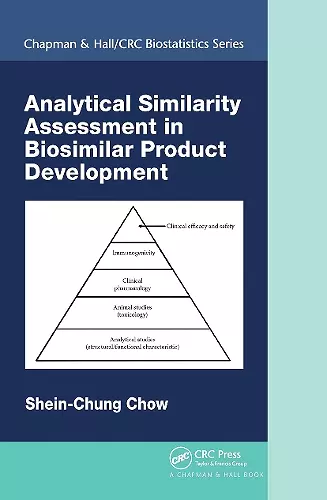
This book focuses on analytical similarity assessment in biosimilar product development following the FDA’s recommended stepwise approach for obtaining totality-of-the-evidence for approval of biosimilar products. It covers concepts such as the tiered approach for assessment of similarity of critical quality attributes in the manufacturing process of biosimilar products, models/methods like the statistical model for classification of critical quality attributes, equivalence tests for critical quality attributes in Tier 1 and the corresponding sample size requirements, current issues, and recent developments in analytical similarity assessment.
ISBN: 9780367733834
Dimensions: unknown
Weight: 650g
340 pages